Your trusted source for contextualizing abortion news. Subscribe to our daily newsletter.
Federal judges have issued contradicting orders about whether mifepristone — one of two drugs used to induce a medication abortion — can be legally distributed.
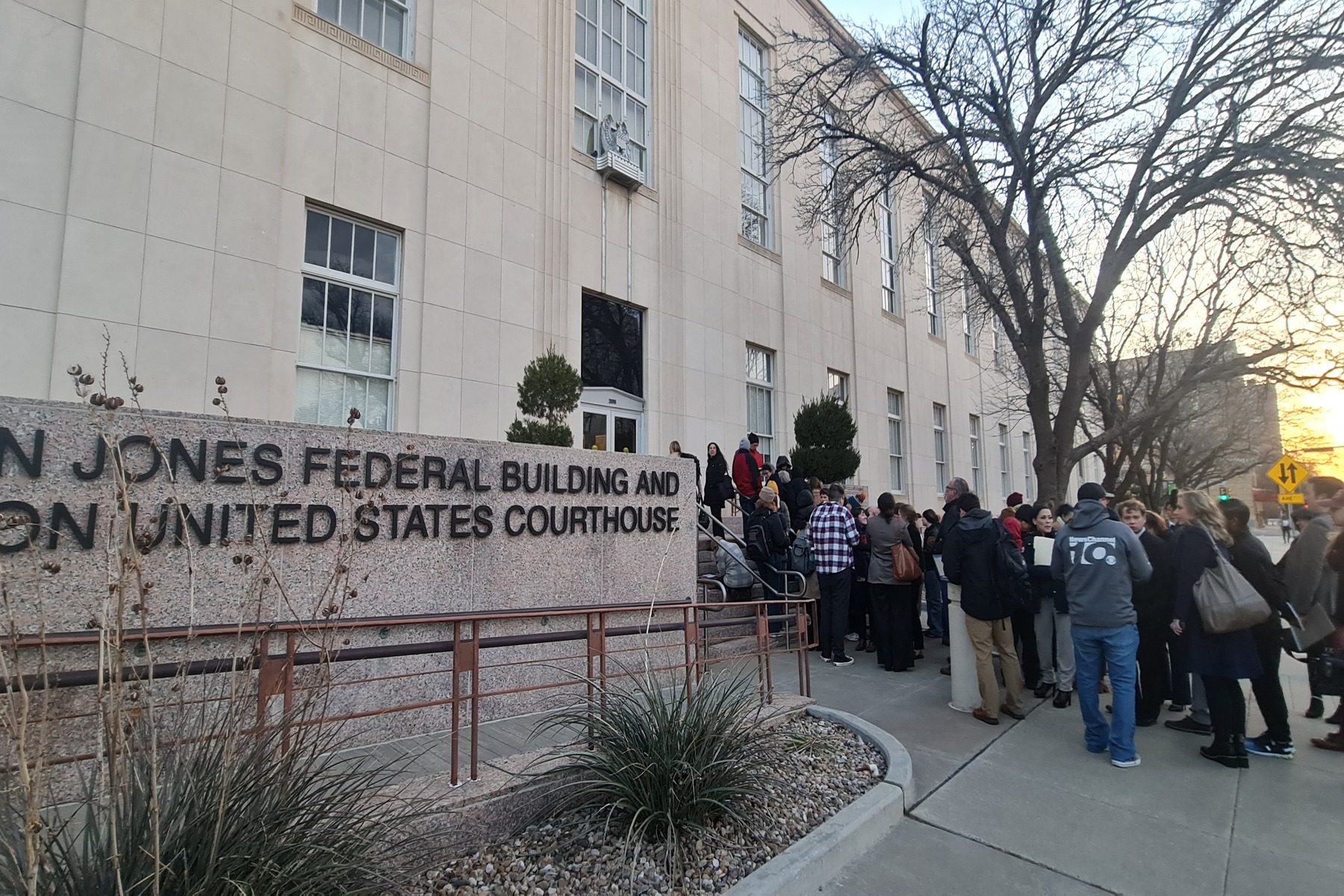
A judge in Texas ruled Friday in a much watched case, saying that the federal government’s approval of the drug must be blocked; his decision, he wrote, takes effect in one week, giving the Department of Justice time to appeal the decision.
But another federal judge, in Washington state, found the opposite in a separate case concerning the drug’s approval. That judge, who also ruled Friday, held that a nationwide injunction blocking mifepristone’s distribution would be “inappropriate.”
The conflicting federal rulings increase the likelihood that mifepristone’s legality will ultimately be decided by the Supreme Court. The stakes for people seeking abortions are significant.
“It’s completely a conflict,” said Laurie Sobel, associate director for women’s health policy with the Kaiser Family Foundation. “I can’t imagine this doesn’t go immediately on the shadow docket to the Supreme Court with two different rulings.”
The Washington ruling only affects mifepristone’s distribution in the 17 states plus the District of Columbia that filed the suit. But protecting mifepristone’s availability in those states directly contradicts the Texas decision, which would apply nationwide.
“The FDA can say we’re not allowed to do this. They’re going to immediately appeal and they’ll say, ‘By the way we’re not allowed to do this order in 17 states and D.C.'”
-
More abortion coverage
- A federal judge could soon block access to an abortion pill. Here’s what that means.
- Senators ask mifepristone manufacturer to list miscarriage as a use for abortion pill
- Label change for mifepristone could reduce barriers to care for miscarriages, advocates say in petition to FDA
If the Texas-based judge’s decision takes effect, people seeking a pill-based option could be forced to use a less effective, at times more painful medical regimen for medication abortions, which is the most common method to terminate a pregnancy. Some clinics told The 19th they will stop providing medication abortions altogether following the ruling. The decision is expected to be quickly appealed.
Medication abortions typically involve two medications: mifepristone, which is administered to stop a pregnancy from progressing, and then misoprostol, which is taken 24 to 48 hours later to empty the uterus. The protocol is only recommended for the first trimester of pregnancy, and has somewhere between a 95 and 99 percent effectiveness rate. The risk of complications is less than 1 percent.
With mifepristone potentially unavailable soon, some abortion providers have been preparing to offer medication abortions using only misoprostol. That regimen — which is used in most other countries, where mifepristone is often unavailable — is safe and effective, but research shows it has a higher failure rate than the two-drug option. (A recent study found misoprostol-only medications had an 88 percent effectiveness rate.) Patients who take only misoprostol also can experience heightened side effects, including greater pain and vomiting. Other providers have said they will distribute mifepristone for as long as they have the drug in stock.
Adjusting to the one-drug regimen would not be easy. Clinicians across the country told The 19th it would take time to adjust to a misoprostol-only protocol. Many clinicians have only ever provided mifepristone-misoprostol regimens up until now, and experts told The 19th that switching to misoprostol only could mean reduced capacity at clinics.
If mifepristone’s distribution is blocked, some providers will now only provide surgical abortions, which are also safe and effective. But surgical abortions can only be offered in clinics, and take more time and resources for abortion clinics to provide.
For patients traveling across state lines to get an abortion, a misoprostol-only abortion presents particular challenges. The higher failure rate means greater odds of needing follow-up care when people have already returned to their home state.
Mifepristone has been on the market since 2000, when it was first approved by the Food & Drug Administration to help induce abortions. Since Roe v. Wade was overturned last summer, allowing states to ban abortion, the mifepristone-misoprostol combination has become even more significant.
Health care providers in states that allow abortion, especially those that have seen a surge in out-of-state patients as states restrict or ban the procedure, have leveraged medication abortion as a way to serve more patients quickly. Medication abortions are cheaper to administer, and patients can safely take the pills from home. Some people who have been unable to leave their home states have ordered mifepristone and misoprostol online to perform medication abortions at home, a practice that is potentially legally risky but is medically safe. (The World Health Organization recommends people taking medications have access to professional medical support if needed.)
The ruling in Texas, issued by Judge Matthew J. Kacsmaryk from the Northern District of Texas, comes in an unusual case. Filed by an anti-abortion group, the lawsuit argues that the government should revoke the FDA’s approval of mifepristone, claiming that the drug was improperly approved. Legal experts have robustly criticized the substance of those arguments.
There is no precedent for a district judge effectively undoing the FDA’s approval of a drug, and it’s not clear if this ruling will spur similar lawsuits. Some reproductive rights advocates worry that Kaczmaryk’s ruling could open the door for challenges to the FDA’s approval of intrauterine devices or emergency contraception pills — methods of birth control that some influential anti-abortion groups also oppose.
Recent polling found that 62 percent of voters disapprove of efforts to block access to medication abortion, including the majority of independents and of women voters.