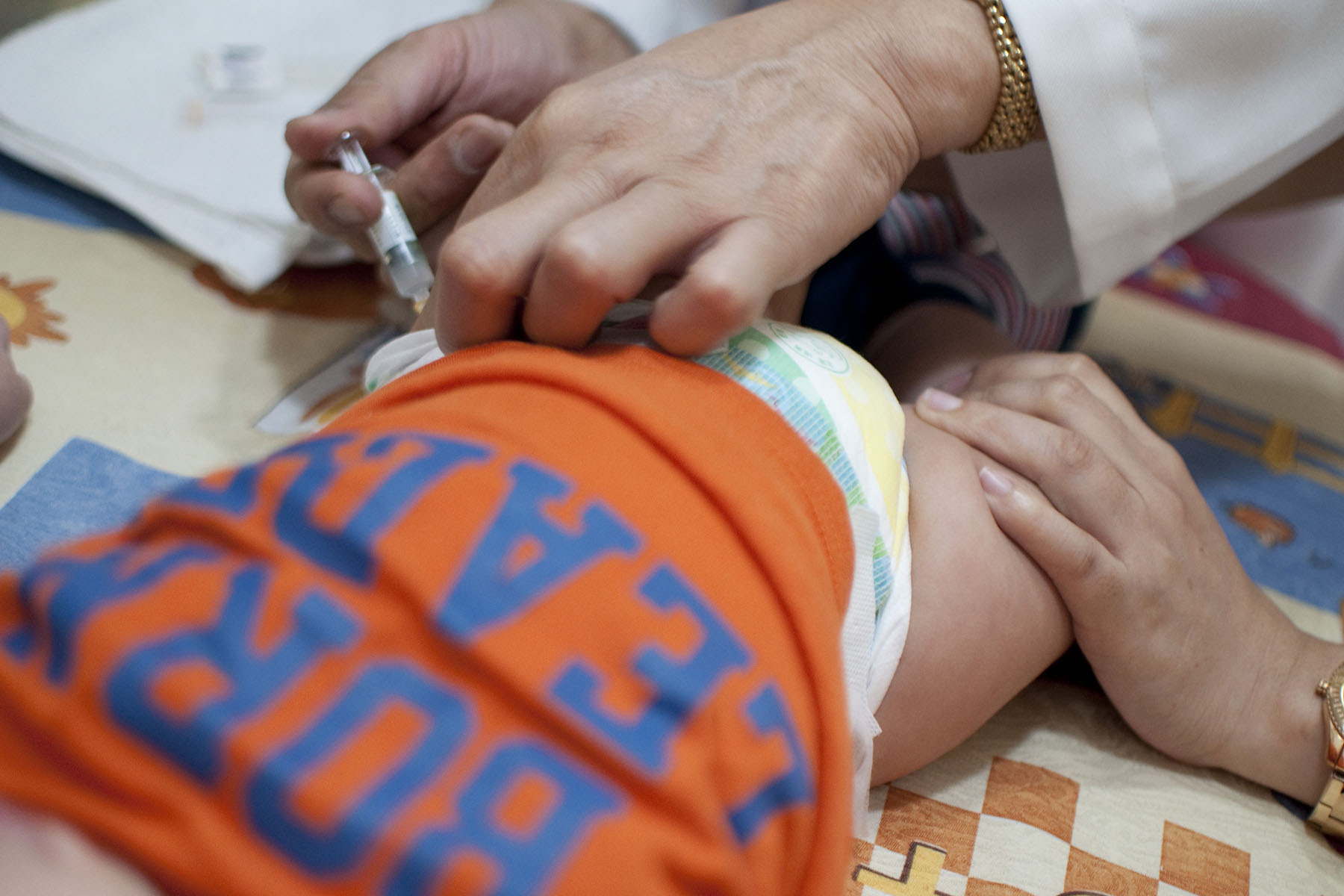
When a new RSV immunization for babies was approved this past summer, Eileen Agosta-Weimer was thrilled. Then pregnant with her first child, she was worried about the virus. She had heard the stories of just how debilitating it could be for infants — and she knew her baby would be at risk of falling ill come winter, when RSV infections typically spike.
Almost immediately, Agosta-Weimer, 42, began to hear from other moms that the shot — known as Beyfortus and approved for use in August — was nearly impossible to find. Parents everywhere were struggling to get their newborns protected. So when she took her son, who was born in September, to the pediatrician for his first round of routine vaccines in November, she made a point to ask: Was there any chance he could get immunized against RSV as well?
She was just in time.
“They had just gotten them in at his pediatrician’s office — it was a Thursday, and they’d gotten them on Tuesday,” she said. “By Monday, they were at a point where they realized the demand was too high. It was higher than they had the supply for. We just lucked out.”
The availability of a new RSV immunization for infants was hailed as a major development for this winter season, especially after 2022’s so-called “tripledemic” of simultaneous flu, COVID-19 and RSV surges. But families across the country have struggled to access the new drug, a monoclonal antibody that provides babies with temporary protection against the virus. Patient and physician demand for the product has far outpaced what manufacturer Sanofi had planned to provide for this year’s RSV season, which typically lasts from about November through March in the United States.
Sanofi has made more immunizations available, releasing another 77,000 doses of the drug this past November, and putting out 230,000 additional ones this week. Those new doses, combined with the initial 1.1 million the company planned to allocate, would be enough to immunize about 40 percent of babies eligible for the drug, according to the company’s estimates. White House officials met with representatives from the drug manufacturer last week to discuss ways to meet patient demand this year as well as next RSV season.
Data from the Centers for Disease Control and Prevention suggests tens of thousands of children younger than age 5 are hospitalized each year because of the virus. Hundreds die. Prior to this year, there was no immunization available for young children, and the new shot can significantly cut the risk of major illness.
But even now, more than halfway through the year’s RSV season, the process of finding the new Beyfortus has remained opaque for the parents tasked with caring for newborns vulnerable to RSV. A vast body of research suggests mothers are more likely to be the ones responsible for making sure their children get immunized.
“It’s always going to be the moms — and sometimes the dads,” said Dr. Kerry Fierstein, a pediatrician from Plainsview, New York. “With RSV, it’s something parents have seen kids suffer. So it makes sense it’s something they would want.”
The majority of pediatricians’ offices ran out of the drug in December, said Dr. Jesse Hackell, who chairs the American Academy of Pediatrics’ Committee on Practice and Ambulatory Medicine. While he is hopeful some will receive more with the new supply available, Hackell — like many other physicians — expressed doubt it would be enough to meet the tremendous patient demand.
Allocating the drug is complicated: It is distributed in both 50-milligram and 100-milligram doses, with the smaller ones earmarked for infants weighing less than 11 pounds. But doctors don’t know in advance how many babies they will see who weigh less than 11 pounds, versus how many would require the larger dose, meaning it’s difficult to predict how many of each shot they should order.
Many immunizations, Hackell said, are now being distributed directly through hospitals for newborns, rather than through individual pediatric practices.
“It’s frustrating not to get it in your office — but it’s good because the highest-risk group is babies,” Hackell said. ”Immunizing babies at birth is the best way to prevent serious disease.”
Beyfortus isn’t the only new product available. Two vaccines were approved this past summer for adults ages 60 and older, who are also at greater risk for serious illness. One of those vaccines — a Pfizer injection called Abrysvo — works for pregnant people as well. People who receive the vaccine between weeks 32 and 36 of pregnancy will pass on antibodies to their newborns, alleviating the need for a separate immunization. As of December 30, 12.1 percent of pregnant women were vaccinated against RSV, a CDC spokesperson said, and pregnant people haven’t reported the same widespread challenges in getting their own shots. (The agency’s data does not account for people who are pregnant but not women.)
Access to the adult vaccine has offered some families relief. But ensuring it protects newborns as well involves perfect timing. When Diana Zimmerman got her RSV shot in October, she was just over 35 weeks pregnant with her second child. She hoped it would ameliorate the need to track down a shot for her baby — Zimmerman had heard the stories about just how difficult it was to find a doctor’s office with the RSV immunization in stock.
Her water broke nine days later. Zimmerman’s daughter was premature but healthy. Still, she hadn’t been in utero long enough after the vaccine to acquire her mother’s antibodies. She would need her own separate protection against RSV.
But Zimmerman, like Agosta-Weimer, happened to be in the right place at the right time. When, five days after giving birth, she had taken her daughter back to the doctor’s office for follow-up care, the pediatrician asked her if she’d like to give her a shot for RSV protection. They had just received their first-ever shipment of the drug. Her daughter was the perfect candidate.
“They gave her the first shot they got in their office,” Zimmerman said. “I happened to be incredibly lucky.”
A Sanofi spokesperson told The 19th that medical providers “will continue to be updated on the status of their orders, any remaining shipments and potential allocation” of the drug. Still, pediatricians say it’s incredibly difficult to know where or how to order RSV immunizations, and whether those orders will be fulfilled.
Fierstein, whose practice sees 30 newborns a month, said she received maybe 20 shots in October. She hasn’t been able to order any more since then, and doesn’t anticipate doing so before the end of the season. Meanwhile, Dr. Michael Sachs, a pediatrician in the Los Angeles area, said his practice hasn’t had any issues receiving the shots, and in fact anticipates having adequate supply to meet patient demand. But he’s not sure what is behind his good fortune.
“Somehow we must have gotten on Sanofi’s ‘good list,’” he said.
Physicians said they anticipate a smoother rollout of the drug next year, especially now that there is a clearer picture of what demand might look like. In time, Hackell said, its impact could be tremendous.
“This product is a game changer,” he said. “This product is an excellent product, and it will prevent a whole lot of severe respiratory disease in the first year of life — hospitalization, even death.”