Your trusted source for contextualizing the news. Subscribe to our daily newsletter.
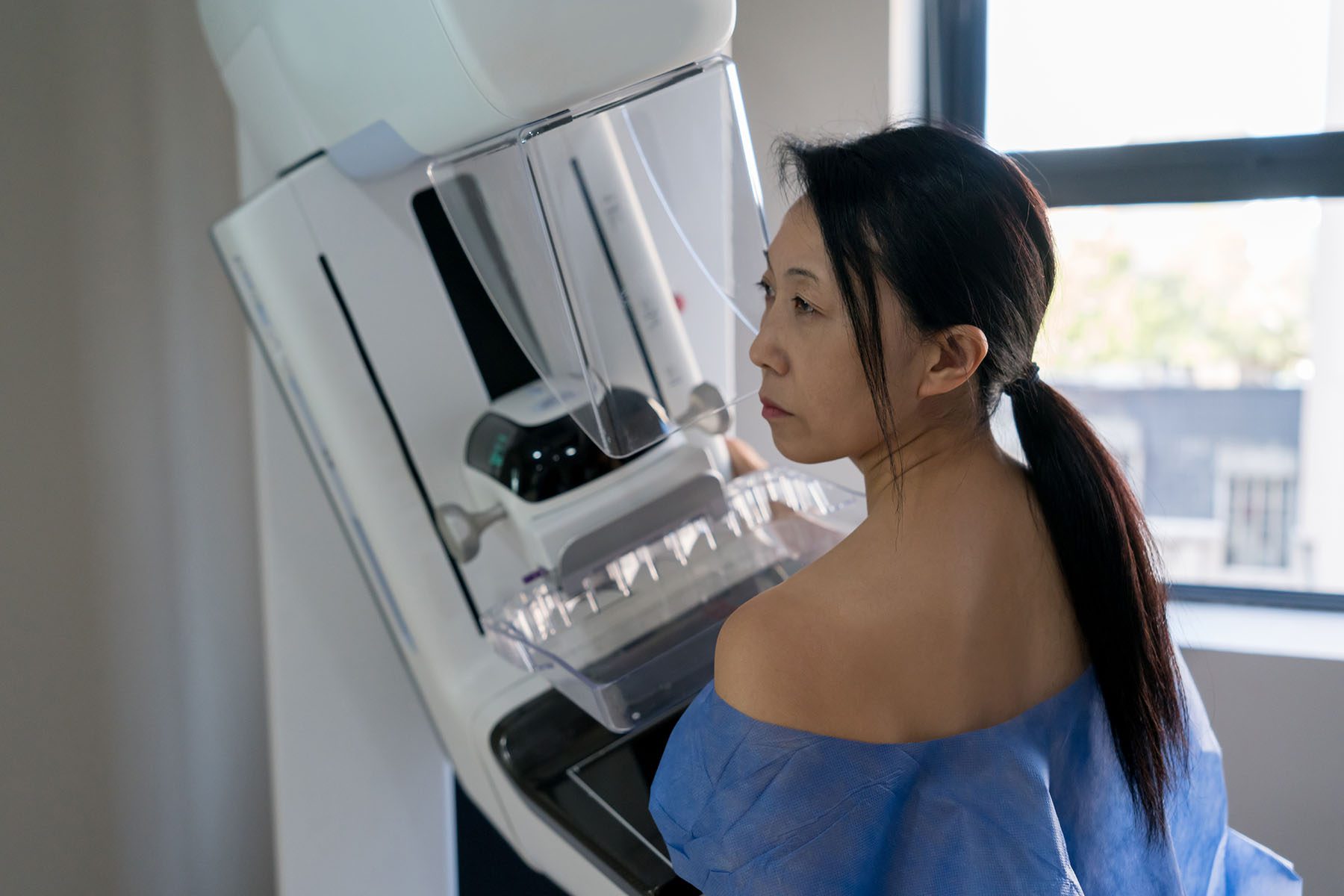
The Food and Drug Administration on Thursday announced new regulations requiring mammogram providers to inform patients about the density of their breasts, a factor that increases the risk of breast cancer and can require additional screenings in order to properly detect the disease.
The new rule comes after a decades-long push for better education and guidance to patients, which advocates say will lead to earlier detection, diagnosis and life-saving treatment. About 50 percent of women in their 40s have dense breasts, in addition to 40 percent of women in their 50s and 25 percent of women older than age 60, according to Dense Breast-Info, a website dedicated to resources about why density matters in breast cancer screening.
The regulation adds to the Mammography Quality Standards Act (MQSA) of 1992, which required the Department of Health and Human Services to develop standards to regulate mammography facilities and services. The addition mandates that providers include an assessment of a patient’s breast density in a mammogram report in order to inform them about the potential limitations of their screening and to enable them to make an informed decision about further testing with their doctor. Providers have until September 2024 to implement any necessary changes to abide by the law.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” Dr. Hilary Marston, the FDA’s chief medical officer, said in a statement.
Dense breasts are classified as those with high amounts of glandular tissue and fibrous connective tissue and lower amounts of fatty breast tissue, according to the National Cancer Institute. Breasts fall into four categories: fatty breast tissue, scattered fibroglandular density, heterogeneously dense and extremely dense. A person’s breast density level can only be determined by a mammogram screening and is primarily determined by genetics and age, Dr. Kirti Kulkarni, a breast radiologist at the University of Chicago Medicine, told The 19th. Younger people, Black people and Asian-American people tend to have more dense breasts, Kulkarni added.
Transgender and gender non-conforming people with breasts can also face risks. Transgender women on hormone therapy have a lower risk of breast cancer than cisgender women, but their risk is higher than if they were not receiving this medical treatment.
Higher breast density can both mask cancer on a mammogram and increase the risk for developing breast cancer. Breast cancer is the second-most common cancer for women after skin, and is the second-leading cause of cancer deaths among women overall, according to the Centers for Disease Control and Prevention.
Cancer is more likely to develop in glandular tissue rather than fatty tissue, which makes dense breasts more at risk. On a regular mammogram — which doctors recommend people start receiving regularly between ages 40 and 50 — highly dense breast tissue appears white, the same as a potentially cancerous mass.
“In extremely dense breasts, a mammogram will miss cancer almost half the time, and they’re four to six times at greater risk of getting breast cancer than those with the least density,” said JoAnn Pushkin, executive director of DenseBreast-Info.
Pushkin was diagnosed with breast cancer 18 years ago, after going through years of mammograms that did not detect any masses. It wasn’t until after she went for an ultrasound that her doctor could see what the mammogram had missed, prompting Pushkin to learn more about the risks of having dense breasts.
“I was just horrified,” Pushkin said. “I had been doing everything I was told was right, I exercised, ate healthy, never missed an annual mammogram, really had no significant family history of breast cancer. But I had extremely dense breasts, so I was just shocked by all of this.”
Following her diagnosis, Pushkin fought for a law passed in New York in 2012 that requires mammography providers to inform people if they have dense breast tissue, the risks associated with dense breasts and options for follow-up screenings. Currently, 38 states and the District of Columbia require some level of breast density notification after a mammogram, she said, but the levels of information offered to patients varies greatly from state to state.
Pushkin first testified before federal officials in favor of the FDA regulations in 2011, but she’s unsure why the effort did not move forward at that time. She commended the agency for making the change now, and said it is important to have a national standard. It remains to be seen how much patients will understand their potential risks and treatment options and how much insurance will cover, she continued.
Pushkin noted that a potential complement to the FDA rule is the Find It Early Act, a federal bill introduced in December by Democratic U.S. Rep. Rosa DeLauro of Connecticut and Republican Rep. Brian Fitzpatrick of Pennsylvania. The proposed legislation aims to ensure all health insurance plans cover screening and diagnostic mammograms and breast ultrasounds and MRIs with no cost-sharing.
For people identified to fall under the two highest categories of dense breasts, Kulkarni recommends that they do additional screenings in conjunction with regular mammograms. This could be an automated whole breast ultrasound or a breast MRI, which is “a robust, more sensitive, more specific test as compared to an ultrasound,” she said. For higher-risk patients, she will also recommend alternating between a mammogram and another screening every six months.
Kulkarni’s state, Illinois, has required patient notification about breast density since 2019, and she said the federal requirement is an important next step.
“I’m happy that the mandate is throughout the country and not just in a few of the states, because people need to get to know their breast density and need to know what the next step is to minimize the size of the cancer,” she said. “The importance is to find these cancers early.”