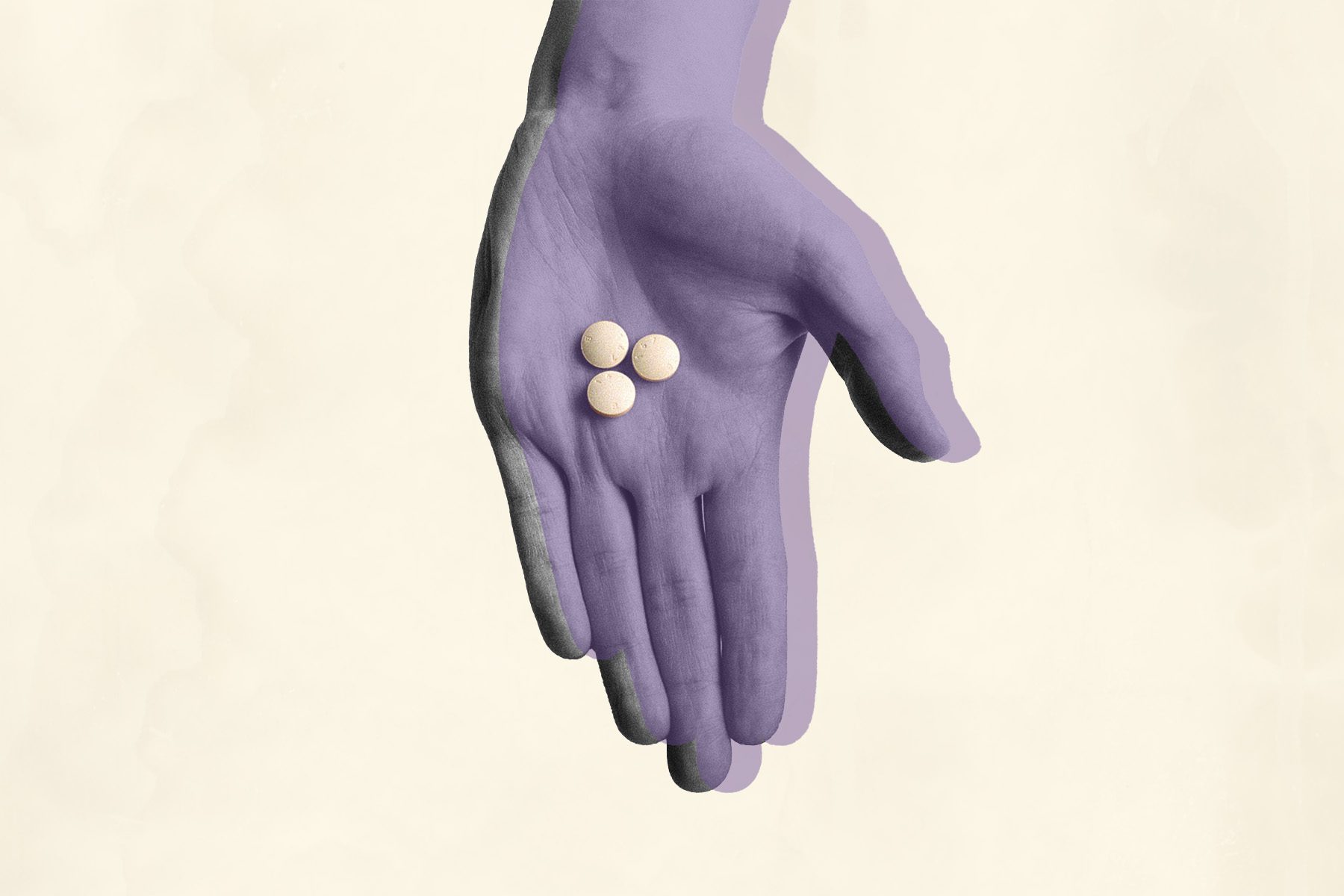
Over 40 medical and advocacy groups submitted a petition to the Food and Drug Administration (FDA) asking for miscarriage management to be added as a use case for mifepristone, a drug commonly used in medical abortions, and ease the restrictions around who can prescribe it.
Groups including the American College of Obstetricians and Gynecologists (ACOG), SisterReach, Physicians for Reproductive Health and the Expanding Medication Abortion Access (EMAA) Project were behind the petition. The changes they asked for Tuesday would make the drug easier to access for people experiencing miscarriages as some doctors and pharmacies have become more reluctant to distribute it after the end of Roe v. Wade.
Mifepristone taken in combination with misoprostol is the most effective regimen for the medical management of miscarriage and medication abortions. Patient access to this drug is currently limited because of both the absence of FDA approval of mifepristone explicitly for miscarriage management and because the drug’s Risk Evaluation and Mitigation Strategy (REMS), the set of restrictions around the drug set by the FDA, limits clinicians’ ability to prescribe mifepristone.
One in 6 known pregnancies globally end in miscarriage, and in unrecognized pregnancies it occurs in about 25 percent of cases. As stated in the FDA petition, miscarriage is also more common among pregnant people who are Black, low income or exposed to environmental pollutants.
From its initial FDA approval in 2000 until December 2021, mifepristone could be dispensed only to a patient in-person under supervision of a clinician who has received certification to prescribe it. Such regulations are in place because of mifepristone’s FDA labeling as a drug indicated for use in medication abortion. Because of this, many medical and advocacy groups say, there is a stigma attached to mifepristone — one that makes the drug harder to access for patients seeking abortion care and miscarriage management care alike.
During the pandemic, the American Civil Liberties Union, on behalf of a group of medical providers, filed suit against the FDA asking that the REMS be lifted, as the in-person dispensing requirement of the REMS made mifepristone impossible to access during lockdown. They were ultimately successful, and patients were temporarily allowed to receive mifepristone by mail or from specially certified pharmacies and take it on their own at home. An FDA policy change in December 2021 made the temporary pandemic modifications to the REMS permanent.
The groups petitioning the FDA say miscarriage management should be added to the mifepristone label because it is the most effective regimen for medical management of miscarriage and has been shown to be safe and effective for use in this case up through the first trimester, during which 80 percent of all miscarriages happen.
“The evidence is clear that mifepristone improves the outcomes of our patients who are experiencing pregnancy loss, minimizing the need for additional interventions during an already traumatic experience at a time when compassionate care is critical for their physical and emotional recovery,” said Dr. Maureen G. Phipps, chief executive officer of ACOG.
Following the reversal of Roe, many states have passed new laws severely restricting, and sometimes fully banning, medication abortion, which uses the same medications — mifepristone and misoprostol — used in medical miscarriage management. Some states have made it illegal for mifepristone to be mailed, others have limited the window during which medication abortion may be utilized, and some states have moved to ban abortion pills outright.
Advocates say that people who need mifepristone for miscarriage also face the same increased risk of potential criminalization. Because people of color and low-income people are also the most likely to be punished or face jail time for seeking abortions, advocates for the label change say this change could help those who need to access mifepristone for miscarriage management without fear of criminalization.
Kirsten Moore, the director of the EMAA Project, told The 19th that the current climate surrounding mifepristone access is one where “confusion plus criminalization equals chaos — which leads to cruelty.”
“The fact that some states are trying to criminalize an FDA-approved drug is something we cannot pretend isn’t happening and these attempts are shutting down the willingness of health care providers to offer this care, whether in the context of abortion or miscarriage, which is amping up the pressure that patients might face as they do their own risk calculus in seeking this care,” Moore said. “As an advocacy community, we needed to make mifepristone’s potential clear.”
While health care providers have used mifepristone off-label for miscarriage management for years, because of the REMS, not all providers can write a prescription for it. Moore said providers will often prescribe a misoprostol-only regimen for miscarriage management in the absence of being able to prescribe mifepristone easily, but patients must take a very large amount of that medication for it to work and experience severe cramping as a result.
“The mifepristone-misoprostol regimen is simply more effective. … We know it’s more effective, we know it’s safe, and anyone going through this experience should be offered this care,” Moore said.
Medical intervention is needed in the case of missed or incomplete miscarriage, when the body has not expelled all of the pregnancy tissue on its own. Without this care, miscarriage can result in hemorrhage, sepsis or death. Studies have shown that medically managed miscarriages conclude faster than miscarriages that happen without any kind of medical or surgical threatmet — often within a few hours if not more than a few days, compared with up to eight weeks. People who start medication management of their miscarriage are less likely to require a later surgical procedure to empty the uterus.
With the current labeling, though, patients often face hurdles in accessing this care.
This past January, Hannah (who asked that her name be withheld for privacy) was thrilled to learn that she was pregnant for the first time. The North Carolina resident and her partner immediately began planning the nursery and traded ideas on parenting philosophies in a Google document. Just weeks after first taking a positive at-home pregnancy test, Hannah suspected she might be miscarrying and went to the emergency room.
Many medical tests later, the viability of Hannah’s pregnancy was determined inconclusive. She was told to go home and to “wait it out” and given the number for a clinic affiliated with the hospital to continue to evaluate the status of her pregnancy.
Just shy of 11 weeks pregnant, Hannah started to hemorrhage at home, and returned to the emergency room where she was treated, but her miscarriage still had not resolved on its own. Hannah asked for medication management to conclude her miscarriage and was shocked when she was told by the ER doctor that though she wished they could give that to her, they could not. She was not legally able to do so because she wasn’t an authorized provider of mifepristone as defined by the REMS.
The doctor, Hannah recalls, said “her hands were tied,” and referred Hannah to another clinic — a specialty family planning provider — who was legally able to prescribe the medication Hannah would need to complete her miscarriage. Hannah called that clinic to make an appointment, and had to wait another four days before she could be seen. Finally, Hannah was able to be seen by a provider there who confirmed that Hannah’s miscarriage was not complete, prescribing her mifepristone.
“It was really deeply disappointing to have to continue to have to deal with the miscarriage when there could have a way to treat it given the harsh physical and emotional symptoms I was having,” Hannah said. “It could have been resolved four days earlier. That might not sound like a lot to someone not having a miscarriage, but to someone having one, that’s awful.”
“I was really mourning this child we thought we were going to have and was no longer going to be entering our lives,” she continued. “I had to prolong the physical process of dealing with it all because, to my view, of a political agenda.”